Which of the Following Elements Has the Highest Electronegativity
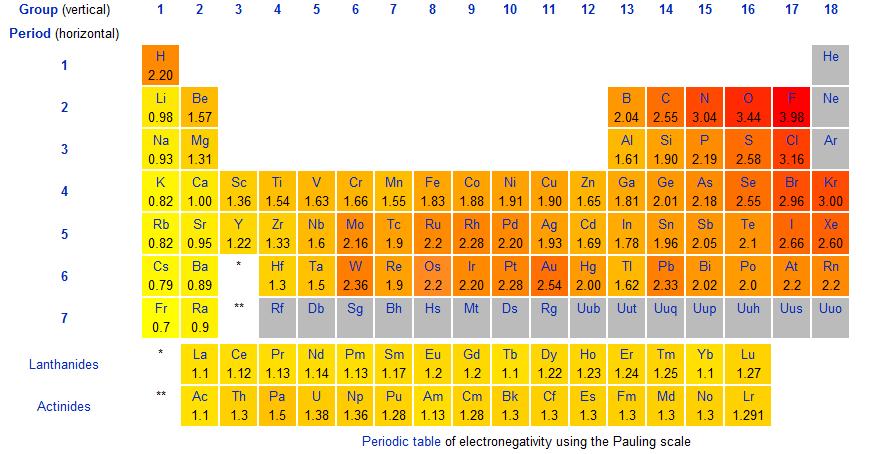
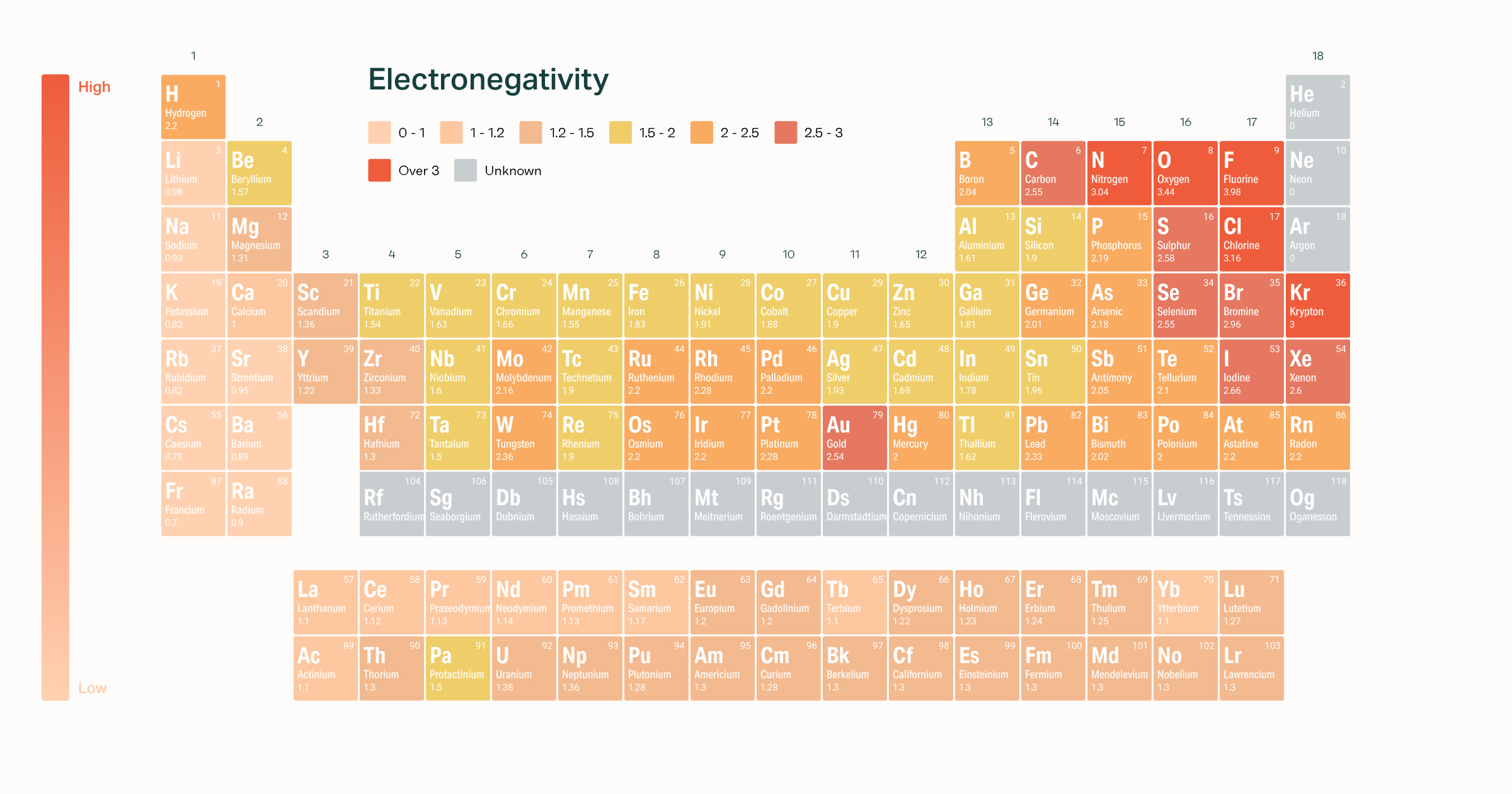
So since elements like the alkaline earths that form cations have low electron affinity it should make sense to you elements that form anions tend toward high electron affinity. Fluorine has the highest electronegativity 40and caesium the lowest 079.

Question Which Of The Following Has The Highest Electronegativity A Na B Br C F Electronegativity Is A Measure Of The Tendency Of An Atom To Ppt Download
It forms only covalent compounds while other.

. The higher the associated electronegativity the. Mg M g e- It is possible to remove more electrons from most elements so. Electronegativity symbolized as χ is the tendency for an atom of a given chemical element to attract shared electrons or electron density when forming a chemical bond.
Element Atomic Radius nm Crystal Structure Electronegativity Valence Cu 01278 FCC 19 2 C 0071. A 01 1 b 11 2 c 10 2 d 13 1. The ionization energy of an atom is the amount of energy that is required to remove an electron from a mole of atoms in the gas phase.
The most commonly used spontaneous fission neutron source is the radioactive isotope californium-252. From left to right across a period of elements electronegativity increases. Throughout the recommendations the use of the electronegativity of elements for sequencing has been replaced by a formal list which is loosely based on electronegativity.
K C Al Si and Ba. Halogens eg iodine chlorine have high electron affinity and also high electronegativity. An easy way to think about it is that the closer an element is to Fluorine the more powerful its electronegativity is.
An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. Iv Absence of vacant d-orbital. Iii Less metallic in character because on moving across a period metallic nature decreases.
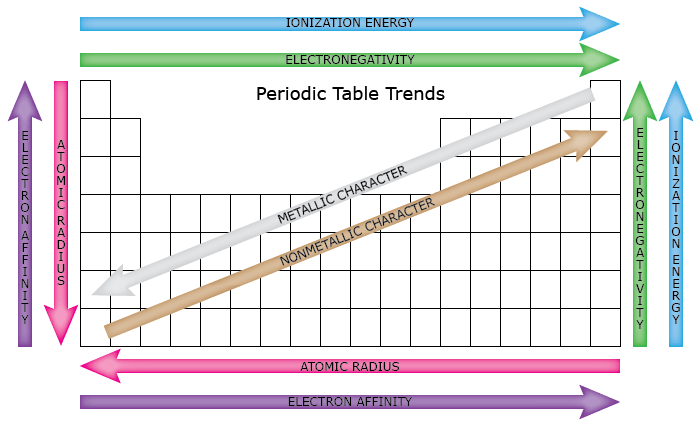
Which Group Has The Highest Electronegativity. A atomic number and therefore charge on the nucleus nuclear or core charge increases b number of valence electrons increases c atomic radius decreases d first ionisation energy increases f electronegativity increases excluding argon g elements on the left are metals elements on. The following general trends are observed as you go across period 3 from left to right.
A simple rule of thumb ignoring lanthanides and actinides is. This means that fluorine. Of the main group elements fluorine has the highest electronegativity EN 40 and cesium the lowest EN 079.
For those that are nonmetals only atomic radii are indicated. The following video shows this. When the difference between.
It shows allotropy while other members do not. Ii Boron has the highest electronegativity because electronegativity decreases down a group. Conversely if the valence shell is more than half full it is easier to pull an electron into the valence shell than to donate one.
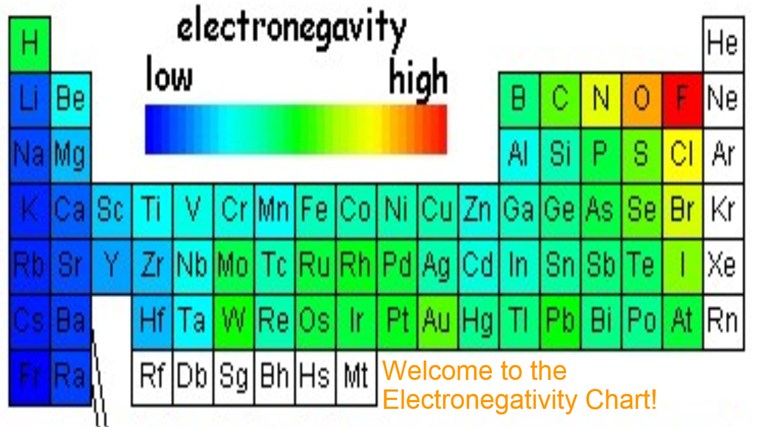
Ionization energies reported in unites of kilojoules per mole kJmol. Which of the following terms accurately describes the energy associated with the process. An electronegativity table of the elements has the elements arranged exactly like in a periodic table except that each atom is labeled with its electronegativity.
340 Sketch within a cubic unit cell the following planes. This indicates that fluorine has a high tendency to gain electrons from other elements with lower electronegativities. Lig Li g e-a electron affinity b binding energy c ionization energy d electronegativity e none of these 5.
It has the highest melting point and boiling point in group 13. Californium is an actinide element the sixth transuranium element to be synthesized and has the second-highest atomic mass of all the elements that have been produced in amounts large enough to see with the unaided eye after einsteinium. From top to bottom down a group electronegativity.
The recommendations still use the terms electropositive and electronegative to refer to an elements relative position in this list. Figure PageIndex1 shows the electronegativity values of the elements as proposed by one of the most famous chemists of the twentieth century. The species that contains 24 protons 26 neutrons and 22 electrons would be represented by the symbol.
Thus the nonmetals which lie in the upper right tend to have the highest electronegativities. Use Mendeleevs periodic table to predict the formula for the oxides of the following elements. A few points of difference are.
Attempting to remove inner core e- kernel e- requires larger amounts of energy. Long Answer Type Questions. Iii Highest electronegativity in the group.
A 50 V 3 b 26 Cr 2 c 50 Cr 2 d 50 Mn 2 e none of these. We can use these values to predict what happens when certain elements combine. It is a non-metal while other members of the group are metallic.
SCPS Chemistry Worksheet Periodicity - page 3 C. If the valence shell of an atom is less than half full it requires less energy to lose an electron than to gain one. Data taken from John Emsley The Elements 3rd editionOxford.
In general electronegativity increases from left to right across a period in the periodic table and decreases down a group. Electronegativity is essentially how many elements want electrons. Because this is where the highest jump in IE occurs following the removal of the valence e-.
Arrange the following elements in order of increasing electronegativity. Crystal structure electronegativity and the most common valence are tabulated for several elements. Electronegativity and Electron Affinity 1.
Which Is The Element In Periodic Table Having Highest Electronegativity Quora

Periodic Trends In Electronegativity Ck 12 Foundation

Question Video Determining Which System Has The Greatest Difference Of Electronegativity Values Nagwa

The Parts Of The Periodic Table

The Parts Of The Periodic Table
What Is Electronegativity Definition Chart And Trends
Electronegativity Chart Of Elements List Of Electronegativity

Electronegativity Boundless Chemistry

Why Does Electronegativity Decrease From Top To Bottom On Periodic Table Quora

Electronegativity Chart Click To Download Free Pdf

Lesson Explainer Electronegativity Nagwa

Electronegativity Table Easy Hard Science

What Is Electronegativity Trends Chart Periodic Table Chemtalk
Electronegativity Of The Elements
Periodic Trends Periodic Table Quiz Quizizz

3 Ways To Calculate Electronegativity Wikihow

Periodic Trends Electronegativity Chemistry For Non Majors
Why Are Noble Metals More Electronegative Then Most Metals Chemistry Stack Exchange
Comments
Post a Comment